.gif) PY4SM
/ PY2DD / ZW4SM
PY4SM
/ PY2DD / ZW4SM.gif)
| Protect yourself: |
| Learn the daily UV Index - click here |
|
Washington - A NASA satellite monitoring detected over Antarctica the largest hole in the ozone layer ever observed, with an area of 28.3 million square kilometers - more than three times the Brazilian territory. The announcement, made today by scientists at the US space agency, indicates that harmful gases to the ozone layer issued years ago are only now causing its further damage. In image captured by instruments aboard the satellite Toms -EP (Total Ozone Mapping Spectrometer Earth - Probe) , the hole appears as a giant blue spot , completely covering Antarctica and reaching Cape Horn , the southern tip of America. Until now, the largest hole in the ozone layer over the South Pole had been registered in 1998, when it was 27.2 million square kilometers, according to NASA. The record hole may have been caused by a change in an air stream spiral over Antarctica - a kind of "jet stream" polar circulating area and contains the ozone hole. This year, the extent of this draft reached further north than normal. Variation Coach Paul Newman, who works with the special spectrometer Toms-EP, said that experts have expected to find a bigger hole this year, but not so great. "I always anticipate some year to year variation, but the hole this year began earlier than usual and is considerably higher than we expected", said Newman. First observed in 1985, the hole over the Antarctic sky is caused by the destruction of the protective layer of Earth's ozone by gases known as CFCs (chlorofluorocarbons), produced by man. Despite being banned since 1987, CFCs remain in the atmosphere - and must stay there for many years, according to Newman. "The peak of vulnerability did not occur immediately after the ban on CFCs," said the coach. "This is after 15 or 20 years." From there, it continues, the situation will return to normal very slowly. "We will be very old people when the ozone hole really disappears". |

- There are many reasons for our concern and among them we cannot disregard the fact that due to photosynthesis, ( triggered by the breaking of the water molecule releasing the oxygen element that "survives about " for just one microsecond ), will certainly find in our flora few species producing ozone, in order that the element oxygen reacts with another atom forming the oxygen gas ( O2 ) and then will react with this same gas thus forming the ozone gas (O3 ); so the Ozone has always been present in the breath of living things and is an excellent bactericide, germicide and anti-virus.
Another important fact regarding the flora is the retention by the organic compounds and consumption of water through perspiration and breathing. This fact is not being taken into consideration by the advance of the oceans on the continents. Therefore, with increasing temperature of the planet we are destroying the flora and consequently invading the continents with water.
You have to evaluate / consider that in a year are involved 204Bilhões cubic meters of water, decomposing 102Bilhões m³ of water, corresponding to a water table of 204km (15.9 times the diameter of Earth or 5 rounds) of long by 1 km wide and 1m high, to secure 72 billion tons of carbon in organic compounds.
To disregard these facts, we are being at least, irresponsible!
The Ozone Layer (O3)
|
The Ozone Layer is a region of Earth's atmosphere, around 25-30 km high, where the concentration of ozone gas is higher. The ozone layer that was formed by photo-dissociation of oxygen gas (destruction of flora with more consumption of oxygen = less oxygen = less Ozone Layer which = less possibility of life) as the Photochemistry theory Sydnei Chapman 1940, matters fundamental to life on planet Earth. Is it that absorbs UV- B radiation from the sun, and so does not allow this radiation harmful to life, reaches the Earth's surface. Radiation in general is the energy coming from the sun. This energy is distributed at various wavelengths. From the infrared to the ultraviolet (UV), through the visible where energy is maximum. In the UV, the UV-C exist, it is totally absorbed in the atmosphere; UV - A, which is not absorbed by the atmosphere; and UV- B, which is absorbed by the Ozone Layer, this being a filter for life. Without it, the ultra- violet rays annihilate all life forms on the planet. |
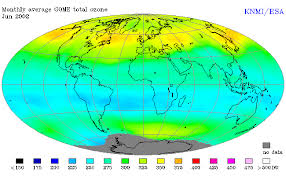 |
The ozone layer is shrinking on an annual average, which amounts to 4% per decade since 1964, and is already reduced by 15% since the beginning of observation. In 1977, British scientists first detected the existence of a hole in the layer over the Antarctic, reaching on 03/09/2000 measurement of 28.3 million square kilometers, or more than three times the size of Brazil. Since 1977, have accumulated records of the layer is becoming thinner in various parts of the world, especially in the next South Pole regions, and recently the North Pole. The destruction of the ozone layer began to worsen in the 30's, when some substances were produced artificially in the laboratory, mainly for applications in refrigeration, these substances being trafficked in some countries where the manufacturing of them is prohibited. Stratospheric Ozone on the northern Europe suffered a record loss of 60% last winter in the Northern Hemisphere, and its total amount of ozone continues to decline, according to a scientific study of several countries.
The UV- B radiation is responsible for numerous sequels in living things. This radiation causes 1.2 million new cases, and kills 12,000 people from skin cancer each year in the United States. Skin cancer is the disease most often mentioned by doctors. But also have undesirable effects on vision, which can produce cataracts and weakening of vision, has negative influence on the DNA of the cells, reducing the body's natural defenses, say people who suffer with herpes, for all we must avoid the sun from 10 to 16 hours, even on cloudy days, in partial or variably as little reduce exposure to UV, and there are people who usually have fever after taking too much sun. It is very white skin, the most sensitive and black skin, the less sensitive.
In the coming decades more ozone will be destroyed, and the UV- B will increase. In 1992, there were cases of fish, sheep and rabbits blind in southern Chile. Doctors in the region have also reported an abnormal occurrence of people with allergies and skin problems and vision. A large area of southern Chile went on alert on 09/10/2000, after the residents have been exposed to violent doses of these rays. Health officials warned the 120,000 residents of Punta Arenas to not expose them to the sun. This can be repeated, reaching, in addition to Chile, Argentina, South Africa, Australia and New Zealand. The Northern Hemisphere is also achieved. The United States, most of Europe, northern China and Japan have lost 6% of the ozone protection.
The United Nations Environment Programme (UNEP) estimates that for every 1 % loss Ozone causes 50,000 new cases of skin cancer and 100,000 new cases of blindness caused by cataracts worldwide. It is therefore important that all take more care. It is a health issue. Who abuse will suffer the consequences. Without the ozone layer sunburn becomes 50 times stronger. In some regions, it has been detected rays UV-B levels five times higher than normal. The consequences of this excessive radiation are tremendous, and the aforementioned reduction in biodiversity, where we find the seriously endangered marine life, especially the plankton (microscopic plants and animals) that live in the sea surface. These tiny organisms are at the base of the marine food chain and absorb more than half of the carbon dioxide (CO2) from the planet.
The Rays Ultra -violet emitted by the sun are endowed with tremendous amount of energy and if not barred in the atmosphere can cause the death of living organisms. Humans are not the only ones affected by ultra- violet rays. All forms of life including plants can be weakened. Among the preventive and educational measures adopted in the United States the creation is included in 1994, the forecast Ultraviolet Index ( UVI ) , distributed nationally, the press of that country. Subsequently, the countries of Europe, Canada and Australia joined the dissemination of the UVI, and now Brazil.
Various chemicals destroy the ozone when they react with it. The blacklist of harmful products to the Ozone layer includes nitric and nitrous oxides expelled by the exhaust of vehicles and the CO2 produced by burning fossil fuels such as coal and oil. The great enemies of the Ozone Layer are substances synthesized in the laboratory, such as bromine and chlorine molecules released into the atmosphere due to industrial goods and technology. The main of these substances are CFCs (chlorofluorocarbons) , HCFCs ( hydro chlorofluorocarbons ) and methyl bromide - present in wide range of products , gases for refrigeration, solvents , foams etc.
These gases tend to accumulate in the colder regions of the globe such as the poles and are for decades or even centuries. So the hole in Antarctica is so great. One component of these substances is chlorine, which attacks and destroys thousands of ozone molecules in the stratosphere. The Hole in the Ozone Layer is a phenomenon that has occurred in Antarctica, that is, in the South Pole region. It is a cyclical phenomenon and a violent destruction of ozone in the atmosphere, during the spring of each year when more than half of the layer It is destroyed. On these occasions, the UV-B radiation increases much. As the layer is the only natural protective filter against UV-B radiation, we must be careful because this radiation tends to increase in the coming years. Scientists say the layer has its constant very long time. The Ozone Layer of the time constant is too large, that is, it will only react to a stimulus after decades. The UV-B radiation is being monitored around the world, including Brazil by INPE.
In the 80's started a real war for the preservation of the Ozone Layer, and one of his greatest victories was the signing of the Montreal Protocol. By this treaty, signed in 1987 by several countries, all substances known to CFC ( chlorofluorocarbon ), responsible for the destruction of ozone, would no longer be mass produced . The ozone layer absorbs the Rays Ultraviolet preventing most of them come to us. The temperature of the stratosphere, mostly maintained by a balance between absorption of solar radiation by ozone and emission of infrared radiation by atmospheric ozone, carbon dioxide and water vapor, so destruction of the Ozone Layer also means , elevated temperature of the planet.
Nuclear tests, the use of supersonic aircraft, and air pollutants endanger the balance of the Ozone layer. Allotrope of oxygen - Oxygen has two allotropes that differ atomicity O2 (oxygen we breathe) and O3 (ozone). Origin and obtaining Ozone: Ozone originates on many occasions , put example in the slow oxidation , the electrolysis of dilute sulfuric acid , the air ionization caused put gamma rays or electrical discharge , by acting light short wavelength (ultraviolet ) or solar ( Photochemistry ) on oxygen or air , acting on oxygen molecule forming two oxygen elements which combine with other molecules also oxygen.
Concern for the Ozone regarding the climate is so large that monitoring of the Ozone Layer is done worldwide, with installed equipment in the earth's surface, satellites and aircraft, using the most diverse and modern techniques, existing the Center Ozone Data World in Ontario, Canada, belonging to the World Meteorological Organization (WMO), based in Geneva, and in Brazil the Ozone Laboratory at INPE.
(Environment Justice x Finance - Flórida, EUA) - On October 4, NASA released by CNN observation of the largest ozone hole ever recorded to date. This was the Morning News, explaining the problems associated with the phenomenon (skin cancers and cataracts), its causes (CFCs ) and how it will take so long for these holes to recover . On the homepage of CNN we can find not only this information, but previous reports, analyzing other holes, other scientific discoveries and other views.
In the afternoon and evening of the same day, excellent reports showed Dr. Paul Newman, in an interview, analyzing the meaning of the hole: as it appears every year in late September, in Antarctica; then breaks into several small holes and spreads through the stratosphere. The cause is always the same: CFCs and other gases. The source has been identified as developing countries that have not yet adopted global treaties that commit countries to eliminate the production of these gases. He also talks about how the observation is made and other very interesting visual details.
The Weather Channel (Chanel Weather) also reported the phenomenon, adding a presentation of ozone molecules and how the UV radiation (UV) part of the molecule and reduces their number, making thinner the cover that protects the earth. All this proceeded (as they were preparing the public) the launch of space shuttle October 5, for another trip planned in series to build the space city (starting as space station) which will be ready in 2005. The station, flights, satellites are the tools that allow us to observe the ozone hole and other phenomena. And NASA has been the generator of such information, with investment from several countries, including Brazil.
The CNN site www.cnn.com has very interesting tool to show the bus and its operation, launch and general information, also in three dimensions. You can click and open the bus, fly it, see it from different angles. Already the site of NASA, www.nasa.gov/ brings answers to several questions, for example, which use the space station when you're ready?
"The International Space Station will have an orbital laboratory for long-term research, where one of the main forces of nature, gravity, is greatly reduced. In addition, global research in biology, chemistry, physics, ecology and medicine may be conducted using the most modern tools available. The medical benefits of doing science in space may generate new drugs and a new understanding of life's building blocks. The effects of micro -gravity eliminate the pressure of gravity in experiments here on Earth. Therefore, treatment cancer can be tested in live cell cultures without risk to patients. The researchers also expect a broad understanding of the effects of human exposure to micro -gravity for a long - time.”
The industrial benefits can generate "chip" computer stronger, lighter and more powerful metals. The absence of convection - the currents that cause the rise of air or hot fluids compared to air or cold liquids that go down - in space will allow different materials to be studied more deeply. Fluids and flames behave differently in micro- gravity.
While some experiments take place inside the spacecraft, others will be made out of it. These experiments help reveal the long - term effects of exposure to the external space environment. Understand phenomena such as extreme temperatures and micrometeorites will help engineers to improve aircraft design. Earth observations allow researchers to study the changes in our natural environment or character caused by humans. Other benefits also will lead to the improvement of weather systems and more accurate atomic clocks. The commercialization of space research will enable industries to explore new products and services. Finally, the result of such innovations will create jobs here on Earth and in space.
|
"We are facing the greatest danger that humanity has ever faced." These
words were spoken by Dr. Mostafa Toba, executive director of the United
Nations Program for the Environment. Then we will see that they are not
exaggerated.
Ozone is a dark blue atmospheric gas, which is concentrated in the stratosphere call, a region located between 20 and 40 km altitude. The difference between the ozone and oxygen gives the impression of being very small; it comes down to an atom: as an oxygen molecule has two atoms, an ozone molecule has three. |
|
This small difference, however, is critical for maintaining all forms of life on Earth because ozone has the function of protecting the planet from the Sun's ultraviolet radiation. Without this protection, life on Earth would be almost completely extinguished. Ozone has always been more concentrated at the poles than at the equator and the poles it is also located at a lower altitude. For this reason, the regions of the poles are considered favorable for monitoring the density of the ozone layer. Since 1957 measurements are made in the ozone layer above the Antarctic and considered normal values range 300-500 dobsons1. In 1982, however, the scientist Joe Farman, along with other researchers from the British Antarctic Survey, first observed strange disappearances ozone in the air over Antarctica. As they were using equipment already an old well, and the data were collecting were unprecedented, given the large decrease in the concentration of gas (about 20% reduction in the ozone layer), saw fit to wait and make new measurements in another time, with a more modern machine before making public a fact as alarming. In addition, the Nimbus 7 satellite, launched in 1978 with the function just to monitor the ozone layer, there was so far detected nothing unusual about Antarctica.
Joe Farman and his colleagues continued measuring ozone in Antarctica in the next two years, the period of spring, and found not only that the ozone layer was still decreasing as though this reduction became bigger and bigger. Now they were using new equipment, which pointed to in 1984, a reduction of 30 % in the ozone layer, a figure confirmed by another earth station located 1600 kilometers away. In the following years the ozone concentration continued to fall at the time of spring and, in 1987, it was found that 50 % of stratospheric ozone had been destroyed before a partial recovery occurred with the arrival of the Antarctic summer.
The Nimbus 7 satellite had not detected the first reductions in the ozone layer for a very simple reason: he had not been programmed to detect ozone levels as low. Values below 200 Dobsons were considered read errors, and so were not taken into account... Scientists could not foresee such a drastic change could take place in the natural order, and therefore had not considered this hypothesis.
In a scientific paper written in 1987, Joe Farman stated: "Before 1985 all the atmospheric chemists thought they were on the right track to understanding ozone observations and the models proposed to harmonize observed and projected changes were less than 1% by decade. However, over Antarctica destruction is now 50 % higher than day, and this for a period between 30 and 40 days each year."
At that time Joe Farman could not even imagine that the destruction still increase more in the coming years, which would widen the hole, that its occurrence would not be restricted to a few days a year, that would appear one second hole in the Arctic and other issues that would arise in globe with decrease in ozone levels.
Indeed, already in 1987 even minor occurrences were detected, dubbed "mini- holes", which appeared near polar region. Even the Antarctic ozone hole variations presented inconceivable that year: in October had disappeared no less than 97.5 % of ozone detected in August, at an altitude of 16.5 km.
In his book "The Hole in Heaven" published in 1988, John Gribbin states that even if there were not detected the ozone hole in Antarctica, the years 1986 and 1987 have already given plenty of reasons for concern. Satellite measurements showed, even then an impressive overall decrease in the concentration of stratospheric ozone around the globe. "This reduction had already reached the southern South America, Australia and New Zealand, the latter with a decrease of 20%. Switzerland also expressed concern at the time when measurements with instruments on the ground revealed a thinning of the ozone layer over the country.
In 1991, NASA announced that the stratospheric ozone over the Antarctic had reached the lowest level so far recorded 110 Dobsons to an expected level of 500 Dobsons. Also in 1991, the United Nations Program for Environment (UNEP) showed that for the first time, was to produce a significant loss of ozone in both the spring and summer, and across the North as in the South, in high and mid-latitudes. This fact has increased the general apprehension, since in summer the sun's rays are much more dangerous than in winter.
In 1992 it was found that there is a hole formed also on the Arctic, with a 20% reduction in ozone. The Arctic hole again not only remained continued to increase: in the first three months of 1996 it grew by more than 30%, setting a new record.
In 1992 the researchers found that the destruction was even more generalized, occurring globally from the Antarctic to the Arctic, the tropics and mid-latitude regions, with a decrease ranging from 10% to 15%. Since that time, the inhabitants of the Falkland Islands / Malvinas began to be exposed to the hole every year during the month of October.
|
The figure shows the variation of the hole in the Antarctic every year from 1979 until 1992. It is observed a continuous growth during the 80s, with a slight reduction of its size in the years 1986 and 1988. Since 1989, however, the hole cannot be reduced further.
In 1995 WMO warned that the hole in the ozone layer in Antarctica had reached the record size of 10 million square kilometers, about the same area of Europe2. Veja magazine of September 1995 reacted this way to the WMO's announcement: "The scene of violent men consumed by skin carcinomas returned to populate the nightmares of the century with the announcement last week by the World Meteorological Organization." In November of that year, also according to the WMO, the hole had the largest area ever recorded for the time of year in a cyclical movement of expansion and reduction: 20 million km². Between September and October 1996, the size of the destruction was of no less than 22 million square kilometers... |
The immediate effect of depletion of the ozone layer is increasing harmful ultraviolet radiation UV- B (see more details below). In 1993, Dr. Paul Epstein, Harvard University, warned that because of increased ultraviolet radiation, the cholera bacillus could be suffering more accelerated mutations, acquiring resistance factors to antibiotics present in giant floating algae blocks in seas.
In 1995, the Scripps Institute of Oceanography in San Diego, California, reported that parts of North America and Central Europe, the Mediterranean, South Africa, Argentina and Chile were already being subjected to significant increases in irradiation ... In 1996 the hole over the northern hemisphere began two months earlier and was the most profound and lasting hitherto observed. In March of that year, the special advisor to the World Meteorological Organization, Romen Boykov, warned: "We are not talking about desert regions, but in populated areas where radiation levels doubled this is very worrying". Boykov made reference now to the observed 45% reduction of ozone in a third of the northern hemisphere. Despite the seriousness of the situation, no matter on the subject was published in the journals Science and Nature, the most important scientific journals in the world. This fact did not go unnoticed to the researcher Jim Scanlon. According to him, investors are very susceptible to news "not optimistic", and the major newspapers seeking filter information that might be considered negative for business. Jim Scanlon said the hole in Antarctica is reported in great detail because it affects relatively few people in isolated areas. But the hole in the Arctic is not reported because it affects about 80 million people in the northern hemisphere.
It is quite possible that Jim Scanlon is right in what he says. I even I could see that some Internet sites allegedly providing scientific data about the depletion of the ozone layer could only be accessed by authorized persons.
Available data in 1996 showed that the annual average ultraviolet radiation in the northern hemisphere was increasing 6.8% per decade, including areas of England, Germany, Russia and Scandinavia. In the southern hemisphere, the growth rate of radiation was 9.9 % per decade, reaching the south of Argentina and Chile. The atmospheric scientist Jay Herman warned: "Increased UV- B radiation is greatest at high latitudes and medium , where most people live and where most of the agriculture takes place". In Brazil, in early 1997, came the news that on the Northeast states ultraviolet radiation level had increased 40 % compared to the same period in 1996...
In March 1997 things got worse. About Argentina and Chile emerged a new hole, dissociated from existing on the South Pole and covering large areas of both countries, including the capital Buenos Aires and Santiago. Measurements of 180 and 210 Dobsons were recorded. According to the Argentine journalist Uki Goñi, the population of Argentina was not properly alerted by Climate Department. The officials said that the episode had "only scientific interest", and that the population should not be alarmed... Goñi also reported that the equivalent latitude in the northern hemisphere would have appeared a similar hole on Washington or Rome.
While the new hole appeared on Argentina and Chile, the pioneer of the South Pole appeared earlier. The ozone began to decline already in March, registering a level of 225 Dobsons; in May the hole over Antarctica was already fully formed. It was the first time this has happened.
In the Arctic the situation was no better. Dr. Pawan K. Bhartia, project scientist TOMS (Total Ozone Mapping Spectromer) warned that they were being detected the lowest values ever measured ozone in March and April: 219 Dobsons. Satellite data indicated that the affected area extended by 5.3 million square kilometers.
As usual, they have started to appear some fancy ideas to solve the growing problem of depletion of the ozone layer on the planet. Russian researchers presented a study according to which it would be possible to repair the ozone layer using laser equipment and satellites. The project consists in setting up a system with 30 to 50 satellites that bombard the atmosphere with ultra-powerful laser beams by stimulating the production of up to 20 million tons per year of ozone; these scientists believe the problem can be solved in ten years, at an estimated cost of 100 billion dollars... There are people who also want to produce ozone on the ground and takes it to the stratosphere rockets, big jets and balloons...
Just based on a sample of all human failures already collected in previous attempts to master, intervene or even predict natural phenomena , we can already say without fear of contradiction , that even if such project were feasible , the end result would be a failure. If it is to encourage such attitudes, exacerbated and unrealistic, it is better to continue presenting other initiatives also innocuous but at least not as costly as the desperate ban on manufacturing CFC and the enactment of the "International Ozone Day", celebrated on September 16 of each year. But what are the effects of the depletion of the ozone layer can bring to the planet and humans in particular? Devastating might be an appropriate adjective.
In 1975, a scientist named Mike McElroy, to study the effects that would come from the destruction of the ozone layer, he warned that this could be used as a new weapon of war. A chemical compound such as bromine, are deliberately released into the atmosphere would lead to a hole in the ozone layer over enemy territory, disabling unprotected people and destroying crops.
If the destruction of the ozone layer has been imagined as a weapon of war, the reader may well get an idea of the effects that will be subject to population and the environment with this event. We managed to perceive with our senses part of the energy emitted by the sun, through the light and heat. But the sun emits energy also outside the range we call visible light, and is therefore not perceived by our eyes. The track "above" the visible light is called infrared range and the "below" it is called ultraviolet. But that does not matter, what matters is to know that rays with shorter wavelengths contain more concentrated energy and is therefore much stronger, or, in other words, much more dangerous.
Nature wisely protected the planet Earth with a shield against harmful ultraviolet radiation. That shield, the ozone layer absorbs much of the dangerous ultraviolet radiation, preventing it reaches to the ground.
All life on Earth is especially sensitive to ultraviolet radiation with a wavelength of 290-320 nanometers. So sensitive, that this radiation is given a special name: UV -B, which means" biologically active radiation". Most of the UV- B radiation is therefore absorbed by the ozone layer, but even the small part that reaches the surface is dangerous for anyone who is exposed to it for longer periods.
All life on Earth is especially sensitive to ultraviolet radiation with a wavelength of 290-320 nanometers. So sensitive, that this radiation is given a special name: UV -B, which means" biologically active radiation". Most of the UV- B radiation is therefore absorbed by the ozone layer, but even the small part that reaches the surface is dangerous for anyone who is exposed to it for longer periods.
In 1995 already it observed an increase in cases of skin cancer and cataracts in the southern hemisphere regions such as Australia, New Zealand, South Africa and Patagonia. In Queensland, in northeastern Australia, more than 75 % of people over 65 have some form of skin cancer; local law requires children to wear big hats and scarves when they go to school, to be protected from ultraviolet radiation. The US Academy of Sciences estimates that only in that country are emerging each year 10,000 cases of skin cancer because of the thinning of the ozone layer. The Ministry of Health of Chile reported that since the appearance of the ozone hole over the South Pole, cases of skin cancer in Chile grew 133%; currently the government has campaigned for the population using protective creams for the skin and not is exposed to the sun during the most critical hours of the day.
Dr. Lerman Signey, Emory University, Georgia, produced a study which states that the reduction of 1 % in the ozone layer would lead, in the US alone, an increase of 25,000 annual cases of cataracts in sight... There estimates indicating that a 50% reduction in the ozone layer around the planet cause blindness and skin burns, blistering within ten minutes.
The UV- B radiation also inhibits the activity of human immune system, the natural defense mechanism of the body. Besides making easier the conditions for the tumors to develop without that the body can combat them, it is assumed that there would be an increase of herpes infection, hepatitis, and skin infections caused by parasites.
Most plants have not been tested for the effects of increased UV- B, but the species analyzed 200 to 1988, two-thirds expressed some sort of sensitivity. Soy, for example, shows a 25% reduction in production when there is a 25% increase in the concentration of UV-B. The phytoplankton base of the marine food chain as well as some fish larvae also suffer negative effects when exposed to a higher UV-B radiation. It has also been found that cattle have an increase of eye diseases such as conjunctivitis and cancer when exposed to a higher incidence of UV-B.
It should be noted that all these effects are caused by a slight increase in UV- B radiation. There is, however, another type of radiation still more formidable: UV -C. The UV-C radiation has wavelengths between 240 and 290 nanometers and is (so far) completely absorbed by stratospheric ozone. It is known that UV -C is able to destroy the DNA (deoxyribonucleic acid) , the basic molecule of life, which contains all the genetic information of living beings . In the words of John Gribbin, "no one can say with certainty what would be the consequences of leaving this radiation to reach the Earth's surface..."
The ozone layer is therefore of crucial importance for life on Earth. Its destruction is equivalent to a reduction of the immune capacity of the planet. Now , at the time of judgment , the human being who lived for millennia unnatural way lost the right to stay protected from harmful effects , whether opportunistic diseases or harmful ultraviolet radiation. AIDS and the reduction of the ozone layer have much in common. Similar effects are at different scales, as the cause of both processes is the same: the intensification of the Last Judgment on Earth. Both events derive from human’s previously existing protection against harmful to health workers. In case the evil ultraviolet radiation in the other opportunistic diseases that attack the organism weakened by the HIV virus which causes AIDS.
The explanation of science, of course, is far from that conclusion. The most accepted theory today is that the ozone hole was caused by human beings themselves, through the continuous emission in the atmosphere of a chemical compound, chlorofluorocarbons, known as CFCs. The chlorine atom of this compound is named as the villain of the piece; some studies suggest that a single chlorine atom can destroy ozone molecules hundred thousand.
Of course, one cannot deny the influence of pollution generated by human beings in environmental imbalances, lavish that he is in defile all that is within their reach. But the magnitude and speed of the destruction of the ozone layer cannot be explained simply by the higher concentration of CFCs in the atmosphere. A Matter of September 1995 Veja magazine on the subject reported that the CFCs fit very well in the chemical model of ozone destruction, and so were left with the taint of blame. "To date, there is no better explanation for the phenomenon, said the report. "
So far, the mathematical models that tried to predict the future decrease of the ozone layer based on the amount of CFCs in the atmosphere failed completely. All of the Nimbus 7 satellite data indicated (up to 1988) that ozone in northern latitudes was disappearing four to six times faster than expected in scientific models.
In addition, none of the models predicted the formation of the holes over the Antarctic and the Arctic, or the reduction of ozone at mid-latitudes. NASA tried to clarify: "The ability of the atmosphere to offset the ozone loss is smaller than we thought."
The fact is that the reduction of the ozone layer cannot be explained by the greater concentration of chlorine in the atmosphere. John Gribbin, for example, while agreeing with the idea of CFC, leaves some questions in the air in his book The Hole in Heaven, as is apparent from the transcript excerpts below:
"Everything fits logically involving chlorine and ClO in the hole of development (although there is very little ClO below an altitude of 16 km, and further studies are required chemistry and dynamics to explain what's going on there). (...) It seems to be telling us [the data collected by satellite] that, ultimately, the destruction of stratospheric ozone has been going twice faster than can be explained by the sum of all effects from CFCs and nitrous oxide by activity solar. (...) No doubt part of it [the reduction of ozone] may be due to changes in the sun. (...) It is possible that changes related effects in solar activity have helped form the special conditions over Antarctica, which have allowed the hole to grow so much in such a short space of time."
In 1997 the per capita consumption of CFCs in developed countries had fallen from 300 grams to 45 grams, and refrigerators and air conditioners have left their factory without CFC. None of this made any difference so far. The assumption changes in solar activity as a cause of the depletion of the ozone layer should not be neglected. We saw in the topic on the Sun, the solar storm in 1972 caused a decrease of more than 10% in the stratospheric ozone concentration. A more detailed study showed that ozone depletion over the North Pole that year was 16%. No one has managed to estimate what would be the effect of another solar flare as 1972 now with the holes at the poles and the continued reduction of ozone in different parts of the globe.
But is this situation so serious, the destruction of the ozone layer, has had the required impact? The impact is undoubtedly greater than in the case of Sun's behavior changes because it is closer phenomenon of humanity. However, as the news so far have appeared quite spaced in time, end up not having the impact it could and should have, if only because the human being does what it takes to forget as soon as possible anything that seems unpleasant.
Below are reproduced some news snippets still the first half of the 90s that, read together, give a clearer idea of the worsening situation:
"The destruction of the ozone layer is no longer limited to Antarctica. From this year, it is also reaching the North of Europe, Siberia, Alaska and Canada. For the first time, this event occurred in the spring and summer. ( . .. ) The document also points to the destruction of ozone in the high and medium latitudes in the Southern Hemisphere (Argentina, Chile, Australia and New Zealand). (...) In temperate regions there is not really a 'hole' in the ozone layer, but several failures, or areas where gas is very thin, frayed as a fabric that passes ultraviolet radiation from the sun."
Some seedlings are showing a deformed development in this southern spring, while certain types of seaweed are secreting a red pigment never seen before.
In Punta Arenas, there is fear and concern around the invisible bombardment of ultraviolet radiation B. Nobody leaves home without protection hats or sunglasses. Doctors have been strongly sought after by patients with allergies and eye and skin irritations."
"A study sponsored by the United Nations provided the first evidence of depletion of the ozone layer over portions of the Northern Hemisphere, including the United States, in the summer period, reported UPI. (...) A NASA report released last April, It showed that the hole in the ozone layer over regions of the United States was increasing at a speed twice higher than previously believed. (...) The situation observed have very grave consequences for marine life, and for humanity, because an increase in ultraviolet radiation reaching the earth can kill phytoplankton, which is the base of the food chain of marine life."
|
|
The above statements should constitute a warning for humans, about one of the most dramatic signs of Judgment Final triggering the gross matter of this earth. In the coming years the news of the destruction of the ozone layer will continue to jump humanity, irrespective of any international agreement to reduce CFCs and other pollutants. No human action, not even the whole will of humanity can change anything about it because it is a karmic return effect on the Law of Reciprocity, which acts now much bolstered by the intensification of the judging irradiation of Judgment."
Notes
- 1. Dobson is the unit that measures the concentration of ozone. It is a
measure of length and indicates the height that would have the ozone layer
if all of it were brought down to sea level pressure and temperature 0ºC. A
Dobson equals one millionth of a centimeter; Dobsons 500 correspond to a
thickness of 5 mm ozone under the described conditions of standard
temperature and pressure.
Reconstitution of the Ozone Layer and decreased greenhouse producing oxygen from carbon dioxide
In 1785, Van Maron, German philosopher, observed the electrostatic characteristics of the air due to ozone. Schombein in 1801, reported the characteristic odor as a new substance, Ozone name, and suggested that the gas occur naturally in the atmosphere. In Germany in 1875, Siemens created the first ozone generator (ozonator). In 1891, it was found that the ozone was able to destroy bacteria in the water. The first experiment using ozone water treatment was carried out in 1893 in Leiden, the Netherlands and the treatment of the Rhine waters. Biologically the Ozone plays the role of an air purifier.
Allotrope: We call allotropy the property that certain chemicals have to form different simple substances. These compounds differ from the other, as the number of atoms. The chemical properties of the allotropic substances are similar; On the other hand, the physical properties are very different, including the amount of energy involved in the transformation of each allotrope and the speed at which these transformations occur. An allotropic form is always more stable than the other: more stably becomes slower is more energetic than the most unstable form. Typically, the most unstable form becomes the more stable (although this process takes millions of years).
Examples: carbon graphite carbon and diamond are the same.
Allotrope of oxygen - Oxygen has two allotropes that differ atomicity O2 and O3.
The ozone gas because of its high oxidation power is a great bactericide, germicide and anti- viral. It is used in water purification and public areas, in so-called ozonizes. There is controversy about the advantages of taking ozonized water, since its bactericidal, germicidal and anti -viral does not distinguish harmful bacteria from those that are essences to our health.
Justification for the use of the Ozonator of areas
The feeling of freshness that is after a storm is due to the transformation of oxygen into ozone gas, which purifies the public areas, promoted lightning hair, and the ozone produced by nature it is part of life of the human race on Earth, which must be determined is the amount of areas ozonated for humans to breathe, because above a certain amount instantly cause burning in the trachea, sensation before this is because the human being is unaccustomed to breathing ozonated public areas or has some illness so what should be avoided is the disease and not the ozone, and the ozone bactericidal, germicidal and anti- viral.
The residual ozone concentration in the drinking water should not exceed 0.4 mg / liter, and the time to not less than 10 minute treatment, ozone being more effective than chlorine for treating waste water leaving no compounds form toxic, destroying resistant microorganisms like cyst of Entamoeba histolytica, the virus that causes hepatitis a ( HAV ) and 99 % of other protozoa and enteric viruses . It is recommended an inhalation limit of 0.1 ppm (parts per million) or 0.2 mg / m³. For the above 0.3 to 0.5 ppm causes toxic effects respiratory mucosa and ocular mucosa, above 1 ppm are highly toxic concentrations, may cause pulmonary edema, inhalation of 50 ppm of ozone in the atmosphere is likely to be dangerous fatal, inhalation of 0.2 ppm put 3 or more hours can cause disturbance of night vision, cause fatigue and loss of motor coordination.
Toxic effects occur mainly in the combination of ozone in concentrations above 0.3 ppm with other substances such as nitrogen dioxide and sulfur dioxide, damaging the ozone layer and can cause visual disturbances, fatigue, fever, bronchitis, memory loss, increased muscle excitability but there are no reports of fatalities, so the reduction of pollutants that make the most toxic ozone and damage the ozone layer is necessary.
The sun rays emitted by the UV are endowed with tremendous amount of energy and if not barred in the atmosphere can cause the death of living organisms. Without the ozone layer sunburn becomes 50 times stronger.
An ozone layer that was formed by photo-dissociation of oxygen gas as the photochemical theory Sydnei Chapman 1940 is at a height 20-30 km from the Earth's surface absorbs the Rays Ultraviolet preventing most of them come to us. The temperature of the stratosphere, mostly maintained by a balance between absorption of solar radiation by ozone and emission of infrared radiation by atmospheric ozone, carbon dioxide and water vapor. Nuclear tests, the use of supersonic aircraft, and air pollutants endanger the balance of the Ozone layer.
Plants can be damaged by ozone when exposed to concentrations as low as 2-5 ppm for 8 hours being: Sensitive plants: Spinach, Tobacco, Alfalfa, Wheat, Oats, Barley, Rye, Orchard grass, red clover, radish, beans, corn, Tomato, Broccoli; Intermediate plants: turnip, parsley, carrot, Petunia, chicory and White Carrot; Resistant plants: Beets, Geranium, Blue Grass, cucumber, cotton and lettuce. As a defense response of plant cells to this oxidative stress generated by ozone, antioxidant proteins can be induced. Atmospheric factors affect the sensitivity of the ozone plant.
Origin and obtaining Ozone
Ozone originates on many occasions, for example in the slow oxidation in the electrolysis of dilute sulfuric acid by air ionization caused by gamma rays or electric discharge, the light acting short wavelength ( ultraviolet) light or sunlight ( photochemical ) on oxygen or on air, acting on oxygen molecule forming two oxygen elements which combine with other molecules also oxygen, as in reaction:
O2 ó 2(O) + 2(O2) ó 2(O2)
Ozone, which is a very active oxidizing agent, is relatively long compared with nascent oxygen that remains, about putting a micro second. We get nowadays almost exclusively put through the so-called dark electric discharge; by passing oxygen or air to put a space subjected to a high voltage , it produces ozone. All normal procedures only give oxygen or ozone air. The pure product is obtained in very low condensing temperatures, so the ozone in the form of a crystalline mass dark blue - violet in color, difficult to handle, and melts at -227 ° C giving a dark blue -violet liquid.
Properties and Applications of Ozone
It is a characteristic odor of gas, almost colorless pale blue, formula O3.
3 O2 + 68,2 Cal.=2 O3
This energy is again released when ozone decomposes, and its oxidizing power greater than that of ordinary oxygen. Ozone was already used in Germany in 1924 in the form of ozonized air, due to its high oxidizing power by which arrives destroying bacteria, germs and viruses , thereby eliminating odors , being applied in the purification of water and air atmospheric . Has a great importance in the technique of drinking water purification, existing in France in 1996, 500 Water- treatment , and in Europe and USA is available in ozonizes market for air purification.
Oxygen production from the Carbon Dioxide (CO2)
Through the dark electric charge due to the electronegativity of carbon ( C ) and oxygen (O) to produce ozone ( O3 ), which is easy to prove observing ozonizes air or high stress points in televisions that after some time use of black display areas is also possible through Photochemistry and gamma radiation, scientific study is necessary for this purpose.
Solutions:
A) Put large ozone generators at hydroelectric plants to harness the ability of excess energy which occurs at night and more frequently during the rainy season.
B ) Place small hydroelectric dead up with purpose to feed ozone generators placed next to them, as there are various operating conditions in Brazil.
C) Build Wind Power Plants and Solar in northeastern Brazil and deserts in other parts of the planet to feed ozone generators for the hot climate of the region collaborated with rising ozone and lower the temperature of the region, and on the equator the concentration lower ozone; this concentration is 240 cm matm (milli atmosphere centimeters).
D) Build hydropower without damming in regions that permits in order to harness the energy to feed ozone generators.
E) Use of ozone as an insecticide and herbicide, requiring scientific study for this purpose.
F) Construction of plants Gamma for air ionization and sterilization of food.
G) Getting the Ozone pollution to rivers.
Comments:
a) These are ways to compensate for nature with deforestation and flooding that have been made with the construction of many power plants, as we will be producing oxygen as ozone.
b) is more than obvious that we are altering the climate with greater intensity in the regions involved and in some first time may be unfavorable, so it becomes necessary for constant observation climate with a view to producing ozone in these regions, possibly requiring the use of filters to chlorine to reduce ozone to molecular oxygen at certain times of year , spring being the highest concentration and the fall of the lower concentration, and in the equatorial region lower average concentration throughout the year, and Ozone content shows a diurnal variation being highest during the evening or at least not smaller than during the day and relative to the ocean concentration is higher in the continent.
The concern of Ozone regarding the climate is such that there is the World Ozone Data Center located in Ontario, Canada, belonging to the World Meteorological Organization and Brazil the Ozone Laboratory at INPE.
The Earth is surrounded by a fragile ozone layer that protects animals, plants and humans from ultraviolet rays emitted by the sun. On the surface, the ozone gas (O3 ) aggravates air pollution of cities and acid rain. But in stratospheric heights (between 25 and 30 km above the surface), ozone is a filter for life. Without it, UV light could annihilate all life forms on the planet.
There is scientific evidence that man-made substances are destroying the ozone layer. In 1977, British scientists first detected the existence of a hole in the layer over Antarctica. Since then they have accumulated records of the layer is becoming thinner in various parts of the world, especially in regions near the South Pole, and recently the North Pole.
Although the ozone layer absorbs most of the ultraviolet radiation, a small portion reaches the Earth's surface. This radiation kills 12 thousand people from skin cancer each year in the United States. Ultraviolet radiation also affects the immune system, undermining the human resistance to diseases such as herpes, in addition to the cancer itself. Cause also eye problems such as cataracts and weakening of vision.
Humans are not the only ones affected by ultraviolet rays. All forms of life including plants can be weakened. It is believed that higher levels of radiation can decrease agricultural production, which would reduce the food supply. Marine life is also seriously threatened, especially the plankton (microscopic plants and animals) that live in the sea surface. These tiny organisms are at the base of the marine food chain and absorb more than half of the carbon dioxide ( CO2 ) from the planet .
Various chemicals destroy ozone when they react with it. Such substances also contribute to global warming, known as the greenhouse effect. The black list of products harmful to the ozone layer includes nitric and nitrous oxides expelled by the exhaust of vehicles and the CO2 produced by burning fossil fuels such as coal and oil. But in terms of destructive effects on the ozone layer nothing compares to the gas group called chlorofluorocarbons, CFCs.
Once released into the air, CFCs take about eight years to reach the stratosphere where, hit by ultraviolet radiation, disintegrate and release chlorine. In turn, the chlorine reacts with ozone which consequently is transformed into ordinary oxygen (O2 - which we breathe). The problem is that the common oxygen is not able to protect the planet from ultraviolet rays. A single CFC molecule can destroy 100,000 ozone molecules.
CFCs are synthetic gases (non-naturally occurring) that are used as propellants in aerosols, as insulation in refrigeration and to produce plastic packaging materials. They are cheap to manufacture and very chemically stable, CFCs have been hailed as substances capable of revolutionizing modern life. But the environmental damage caused by CFCs is forcing the industry to look for other alternatives.
The hole that comes with spring
A number of climatic factors make the stratosphere over Antarctica an especially susceptible to ozone depletion region. Every spring in the Southern Hemisphere, usually in October, appears a hole in the ozone layer over the continent. The scientists observed that the hole is growing and that its effects have become more evident. In 1992, there were cases of fish, sheep and rabbits blind in southern Chile.
Doctors in the region have also reported an abnormal occurrence of people with allergies and skin problems and vision. The Northern Hemisphere is also achieved. The United States, most of Europe, northern China and Japan have lost 6% of the ozone protection. The United Nations Environment Programme (UNEP) estimates that each 1% ozone loss causes 50,000 new cases of skin cancer and 100,000 new cases of blindness caused by cataracts worldwide.
To save the layer
Several countries have been working to eliminate the production and use of CFCs . In 1989 came into force the Montreal Protocol, which provided, through gradual measures, the elimination of CFCs. The countries that initially joined the agreement represented 82 % of world consumption of CFCs. The Protocol has been frequently updated and contains provisions to meet the needs of developing countries, whose consumption is still low. Currently, 155 countries are signatories to the agreement.
From 1988 to 1992, the consumption of CFCs fell 40%. In December 1995, the European Community and the United States almost completely banned the production and import of CFCs and other substances harmful to the ozone layer. To achieve this, one of the measures adopted was the recycling of gases ever produced, especially in electrical equipment. The industry of these countries could also produce aerosols that use innocuous alternative propellants for the ozone layer. However, rising prices of CFCs in these countries have stimulated the emergence of a black market for the product, smuggled from countries where production of these substances is still legal.
Search for alternatives
You can replace CFCs by other synthetic products, although its effectiveness and its effects on the environment are still uncertain. The HFCS product (CFCs with an additional hydrogen atom), for example, cause less damage to the ozone layer. But such a variety gas, HCFC 142b is flammable and others that are toxic.
The HCFC 134a (mainly used as aerosol propellants and foam manufacturing cosmetics) is considered safe to humans but apparently less effective as a cooling agent than conventional CFC. Therefore, a refrigerator that uses HCFC 134a spends more electricity to maintain the same temperature. For countries that rely on fossil fuels to generate electricity, it could contribute to the greenhouse effect. Thereby solving a problem may worsen another.
The search for alternatives to CFCs must continue to ensure the complete removal of these gases. It is also vital to promote technical cooperation with the richer countries to ensure that all nations adopt new technologies. After all, the ozone layer protects the planet.
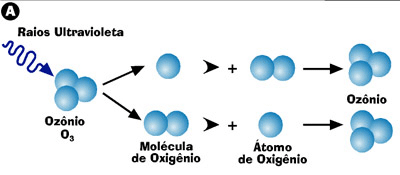
Formation and destruction of ozone
(A) In the atmosphere, the presence of ultraviolet radiation triggers a natural process that leads to continuous training and fragmentation of ozone.
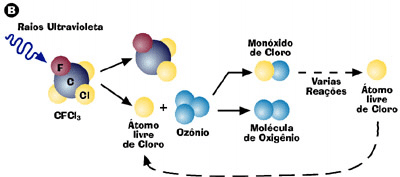
(B) A breakdown of CFC gases is harmful to the natural process of formation of ozone. When one of these gases (CFCl 3) fragments, a chlorine atom is released and reacts with the ozone. The result is the formation of an oxygen molecule and a chlorine monoxide molecule. Later, after a series of reactions, a further chlorine atom will be released and will return to again trigger the destruction of the ozone.
![]()
![]()