Mercury battery replacement
There used to be a time when mercury oxide batteries were available. These
batteries had some significant advantages: One was that they had the highest
capacity per size for their time, and another was an extremely stable output
voltage. But they also had a significant disadvantage: They were highly
toxic, so they required careful waste disposal.
But those times were similar to ours in one aspect: You cannot trust
all people to behave intelligently. Instead of bringing their worn mercury
batteries to disposal centers, people simply discarded them in the household
trash. So the poison was ending up in the soil, in the rivers, in our drinking
water.
These times have ended, as our rulers in their infinite wisdom have
found it suitable to eliminate the mercury battery waste disposal problem
by eliminating mercury batteries. So, their manufacture, sale and use has
been banned in almost all countries of the world, and while small stocks
of these batteries remain, they are ever harder to get by, and their prices
are reaching collector's levels. Alternatives are needed.
The most commonly used mercury battery was one known as PX625, or several
other designations. It's an odd-shaped cell that reminds a little of a
flying saucer. This battery was used in many cameras, light meters, and
some other equipment made in the 1960s and 1970s. The light meters (both
the standalone ones and those built into cameras) usually employ the highly
stable 1.35V delivered by these batteries, to directly power a meter through
a light sensitive cell. If the battery voltage varies, the meter will give
a wrong measurement. So we need to power these devices from a stable 1.35V,
generated from some kind of battery we can find.
Battery manufacturers have come out with sort-of-replacements that have
the same shape as the PX625, but are based on alkaline zinc-manganese chemistry.
Usually they are called PX625A. These cells nominally are rated at 1.5V,
but the voltage varies a lot, from about 1.6V when new down to 1V at what
is considered the end of their useful life. These batteries can power cameras
that don't require a stable voltage, which is common with cameras that
used two or three cells. Due to the lower capacity of alkaline cells compared
to mercury cells, they will last about half as long as the originals. But
the single-cell cameras normally use the voltage directly, and use of the
alkaline replacements will result in wrong exposure.
Reportedly silver oxide replacements for the PX625 exist too, but I
haven't come across them. It seems that most people wanting to use silver
oxide cells in their cameras made for PX625 cells end up making or buying
an adapter for SR44 or equivalent cells. Using silver oxide cells will
of course also give wrong exposure, but at least the exposure will be off
by roughly the same amount throughout the lifetime of the cell...
Solutions:
Zinc-Air batteries: They have been around for a long time. They
deliver a voltage that is close enough to that of the mercury battery to
be used directly, and this voltage is pretty stable too. Their capacity
is high, so there are no problems with this. The big trouble is that these
batteries need to be activated before use, and once activated, they last
only for a relatively short time before drying out, regardless of whether
they are used or not. This time is typically 1 month, which is uncomfortably
short for most camera users accustomed to 3 or 5 years of service from
a mercury battery. Special zinc-air batteries have appeared, which use
less ventilation and thus last longer, up to about three months, which
is still short for many applications. But professionals who use their gear
a lot may become happy with this solution, specially considering that zinc-air
batteries are quite cheap.
Silver oxide batteries with dropping diode: A silver oxide cell
produces a nominal 1.55V. This voltage is relatively stable, but not as
much as that of a mercury cell. It's typically 1.65V for a new cell, while
the end of life is usually rated at 1.35V. Some people use these cells,
and connect them in series with a Schottky diode, in the hope that the
diode will drop about 0.2V, ending up with the correct voltage. This is
a very poor solution, for three reasons:
- The voltage becomes highly dependent on load current, because the
voltage drop across a diode is not at all a constant value, but a logarithmic
function of current. A quick test with an SB160 Schottky diode connected
to 1.55V produced 1.29V at a load current of 1mA, and 1.52V at a
very low load (the current needed by the voltmeter employed for the test).
- The voltage is temperature-dependent, because the diode voltage drop
depends on temperature.
- The output voltage at a given current and temperature is a lot less
stable over the life of the cell, then it would be for a mercury cell.
This is due to the combination of two facts: That the silver oxide cell
is less stable, and that it has higher voltage, a fixed part of
which (not a proportion!) is dropped by the diode. So, if the silver oxide
cell drops its voltage by 10%, the output voltage will drop by 11.5%.
I would consider the Schottky diode solution only in situations where
the current demand of the camera is fairly constant, and then only if I
can find a Schottky diode that will drop the proper amount of voltage at
this specific current level.
Voltage regulator: The most obvious solution would be a voltage
regulator producing a clean 1.35V from an alkaline or silver oxide battery.
But there are a few problems with this approach:
- A regulator will always need some current for its own use. This current
can be a small percentage of the output current, but if the output is required
only a minute a day, and the regulator stays connected 24 hours a day,
the amount of battery drain caused by the regulator is significant.
- The circuit requires several electronic parts, and is too large to
install inside the battery holder. It becomes necessary to build it into
the camera.
In situations where these two disadvantages seem acceptable, a voltage
regulator may be the best approach to the mercury battery problem.
My regulator
In the desire to help the many people who are troubled by the unavailability
of mercury batteries, I have designed, prototyped and tested a little regulating
circuit that could be used in many cameras and meters. It employs only
very easily available components: 6 standard value resistors, and 3 small
transistors, which can be chosen among many.
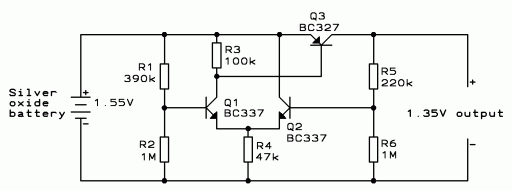 This regulator
is suitable for devices that need up to 1mA. For higher output current,
the resistors would have to be scaled down in reverse proportion to the
peak current expected. With the resistor values shown, the regulator draws
a current of only 0.015mA, which means that a typical SR44 silver oxide
cell would last for about one year and three months powering it. Adding
the current actually consumed by the camera, you might expect a typical
battery lifetime of close to one year. The output voltage measured in the
lab varied from 1.39V for zero load, to 1.34V at a load of 1mA. This seems
acceptable even for the most critical applications.
This regulator
is suitable for devices that need up to 1mA. For higher output current,
the resistors would have to be scaled down in reverse proportion to the
peak current expected. With the resistor values shown, the regulator draws
a current of only 0.015mA, which means that a typical SR44 silver oxide
cell would last for about one year and three months powering it. Adding
the current actually consumed by the camera, you might expect a typical
battery lifetime of close to one year. The output voltage measured in the
lab varied from 1.39V for zero load, to 1.34V at a load of 1mA. This seems
acceptable even for the most critical applications.
The silver oxide cell provides the reference voltage, so the output
voltage will vary in proportion to the cell voltage. For this reason, the
actual output voltage will vary over the life of the cell. But in any case,
it will be a lot better than using a Schottky diode!
It would seem attractive to include a real voltage reference in the
circuit, so that it could be used with an alkaline cell and keep the output
voltage constant even when the cell drops. The problem is that it's not
easy to implement a stable voltage reference that has very small internal
current consumption, specially when the available input voltage is as low
as here! Such a circuit would probably be feasible only when there is a
power switch between the battery and regulator, so that the higher consumption
of the regulator is no longer that important.
If you want to build this circuit, here are some hints:
- The transistors I used are by no means optimal. I used them simply
because I had them lying around on the desk. If you want to look for better
ones, Q1 and Q2 are small signal NPN transistors that should have an Hfe
as high as possible, at very low collector current. These transistors are
working at only some microamperes of collector current, which should mean
a base current of only a few tens of nanoamperes! Q3 is a small signal
PNP transistor, that also should have a high Hfe at low current levels
and low collector-emitter voltage, but it is less critical than the others.
The leakage current for all three transistors must be as low as possible.
The transistors may not be Darlingtons, because these need too much base-emitter
voltage and cannot saturate low enough.
- The resistors can be of the absolutely smallest power rating you can
find. In fact, R4, which is the one having the highest dissipation, works
at a level of around 5 microwatts!
- In most cases you will want to build this circuit into the camera.
So, it's a good idea to look for the tiniest surface mount resistors and
transistors you can find. Even if the old cameras that used mercury cells
usually are not too crammed inside, it helps if the circuit you have to
add is tiny!
- Use good, clean assembly techniques. If you have leakage paths through
solder flux residues or such, they can easily upset the operation of this
circuit.
- I did not include any filtering/decoupling capacitors. If you feel
better doing so, you could add them across the cell, across R2, and possibly
across the output. The first two could be 10 to 100nF, but across the output
I would use a smaller one, perhaps 1nF. In any case, here in my rather
noisy workshop environment the circuit worked well without such capacitors.
This regulator is certainly not a universal solution, but may be a
good one for some applications. I hope that it may help some of you rescue
a nice old camera!
Back to homo ludens electronicus.
 This regulator
is suitable for devices that need up to 1mA. For higher output current,
the resistors would have to be scaled down in reverse proportion to the
peak current expected. With the resistor values shown, the regulator draws
a current of only 0.015mA, which means that a typical SR44 silver oxide
cell would last for about one year and three months powering it. Adding
the current actually consumed by the camera, you might expect a typical
battery lifetime of close to one year. The output voltage measured in the
lab varied from 1.39V for zero load, to 1.34V at a load of 1mA. This seems
acceptable even for the most critical applications.
This regulator
is suitable for devices that need up to 1mA. For higher output current,
the resistors would have to be scaled down in reverse proportion to the
peak current expected. With the resistor values shown, the regulator draws
a current of only 0.015mA, which means that a typical SR44 silver oxide
cell would last for about one year and three months powering it. Adding
the current actually consumed by the camera, you might expect a typical
battery lifetime of close to one year. The output voltage measured in the
lab varied from 1.39V for zero load, to 1.34V at a load of 1mA. This seems
acceptable even for the most critical applications.