|
Back to the SARA Home Page |
||
|
|
||
|
Next S.A.R.A. Meeting on Thursday, May 15th at the Doylestown This Month's Meeting will include: Field Day Plans |
||
|
|||||||||

|
|||||||||
|
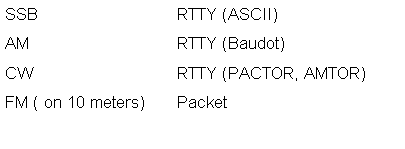
The HF Bandplan - What Frequency to Use? Last month, we examined the bandplan for the 2 meter amateur band. What about 160 through 10 meters? That's a lot of spectrum, and is shared by a great number of users, using a great number of modes. To make matters worse, the HF spectrum is shared with users around the world. |
||
|
compatible with each other: |
||
|
with broadcast stations such as BBC, Radio Netherlands, and others throughout Asia, Africa, and Europe. |
||
|
DARWIN AWARD WINNER FOR 1997 ANNOUNCED In 1996 the winner was an air force sergeant who attached a jet engine (JATO) unit to his car and crashed into a cliff several hundred feet above the road. And now, the 1997 winner: Larry Waters of Los Angeles-one of the few Darwin winners to survive his award-winning accomplishment. Larry's boyhood dream was to fly. When he graduated from high school, he joined the Air Force in hopes of becoming a pilot. Unfortunately, poor eyesight disqualified him. When he was finally discharged, he had to satisfy himself with watching jets fly over his backyard. One day, Larry, had a bright idea. He decided to fly. He went to the local Army-Navy surplus store and purchased 45 weather balloons and several tanks of helium. The weather balloons, when fully inflated, would measure more than four feet across. Back home, Larry securely strapped the balloons to his sturdy lawn chair. He anchored the chair to the bumper of his jeep and inflated the balloons with the helium. He climbed on for a test while it was still only a few feet above the ground. Satisfied it would work, Larry packed several sandwiches and a six- pack of Miller Lite, loaded his pellet gun-figuring he could pop a few balloons when it was time to descend-and went back to the floating lawn chair. He tied himself in along with his pellet gun and provisions. Larry's plan was to lazily float up to a height of about 30 feet above his back yard after severing the anchor and in a few hours come back down. Things didn't quite work out that way. When he cut the cord anchoring the lawn chair to his jeep, he didn't float lazily up to 30 or so feet. Instead he streaked into the LA sky as if shot from a cannon. He didn't level of at 30 feet, nor did he level off at 100 feet. After climbing and climbing, he leveled off at 11,000 feet. At that height he couldn't risk shooting any of the balloons, lest he unbalance the load and really find himself in trouble. So he stayed there, drifting, cold and frightened, for more than 14 hours. Then he really got in trouble. He found himself drifting into the primary approach corridor of Los Angeles International Airport. A United pilot first spotted Larry. He radioed the tower and described passing a guy in a lawn chair with a gun. Radar confirmed the existence of an object floating 11,000 feet above the airport. LAX emergency procedures swung into full alert and a helicopter was dispatched to investigate. LAX is right on the ocean. Night was falling and the offshore breeze began to flow. It carried Larry out to sea with the helicopter in hot pursuit. Several miles out, the helicopter caught up with Larry. Once the crew determined that Larry was not dangerous, they attempted to close in for a rescue but the draft from the blades would push Larry away whenever they neared. Finally, the helicopter ascended to a position several hundred feet above Larry and lowered a rescue line. Larry snagged the line and was hauled back to shore. The difficult maneuver was flawlessly executed by the helicopter crew. As soon as Larry was hauled to earth, he was arrested by waiting membersof the LAPD for violating LAX airspace. As he was led away in handcuffs, a reporter dispatched to cover the daring rescue asked why he had done it. Larry stopped, turned and replied nonchalantly, "A man can't just sit around." |
||
|
|||||||||
|
Will the Lithium-Ion Battery Prepare the Way for the 21st Century? A few years ago, the nickel cadmium (NiCd) was the only battery suitable for use in portable radios, cellular phones, laptop computers and video cameras. To satisfy the demand for increased run-time, new battery chemistries that provide twice the energy densities have emerged. One such battery is the lithium-ion (Li-ion). Will the Li-ion eventually replace the classic NiCd? The answer is no - at least not for now. Every invention that solves one problem creates new ones. By packing more energy into a cell, characteristics such as load current, ease of use, and cycle-life are often adversely affected. When designing a battery for maximum energy density, the cost of energy storage is often ignored. Whereas the kilowatt-hour (kWh) of a well-maintained NiCd is $7.50, the equivalent of a Li-ion is $55. (In comparison, the cost of a household kWh is 5-10 cents). In applications where energy density is of utmost importance, however, operating cost is secondary. One major advantage of the Li-ion is the absence of memory. Memory means that a battery can remember how much energy was required on the previous application. No scheduled cycling is required to prolong the battery life, a benefit that compensates for its higher operating costs. Self-discharge is less than half of the NiCd, making the Li-ion better suited for fuel gauge applications. Compared to the mature, rugged and well-under-stood qualities of the NiCd battery, the Li-ion is fragile and requires a protector circuit to keep the battery within safe operating limits. The charging process is critical and does not allow for methods other than those specified by a manufacturer. The Li-ion is not as forgiving as the NiCd, if abused. Li-ion's History Pioneering work for lithium batteries began in 1912 by C.N. Lewis, but they were not commercially available until the early 1970s. Attempts to develop rechargeable lithium batteries followed in the 1980s, but subsequently failed. Lithium is the lightest of all metals, has the greatest electrochemical potential, and provides the largest capacity. Rechargeable batteries using lithium metal as an electrode are capable of providing both high voltage and excellent capacity, resulting in an extraordinary energy density. However, research concluded that occasional shorts from lithium dendrites cause thermal run-away. The cell temperature quickly approaches the melting temperature of lithium, resulting in violent reactions. In 1991, 10,000 rechargeable lithium batteries sent to Japan had to be recalled after a battery exploded in a cellular phone and burned a man's face. Because of this inherent instability, especially during charging, research shifted to a non-metallic lithium battery using lithium ions from chemicals such as LiCoO2, the chemistry of most commonly used Li-ion batteries. Although slightly lower in energy density than the lithium metal battery, the Li-ion battery is safe, provided certain precautions are met when charging and discharging. In 1991, Sony was the first company to commercialize the Li-ion and is presently its largest supplier. |
||
|
Each battery pack must be equipped with a control circuit to limit the cell voltage peak during charge and prevent the voltage from dropping too low on discharge. In addition, the maximum charge and discharge current must be limited and the cell temperature monitored. If these precautions are taken, the possibility of metallic lithium plating occurring due to overcharge is virtually eliminated. |
||
|
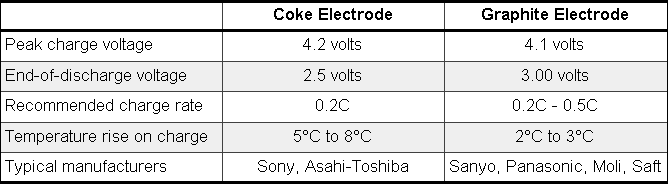
Two basic Li-ion types have emerged: the "coke" version by Sony and the "graphite" version from most manufacturers. |
||
|
|||||||||
|
Manufacturers are constantly changing and improving the chemistry of the Li-ion battery. Although the negative electrode uses carbon, not all desirable characteristics appear with one carbon type alone. New carbons that theoretically double the capacity of the Li-ion battery are being tested. |
||
|
Since higher voltage thresholds provide higher capacities, it is in the manufacturer's best interest to choose the highest voltage threshold possible without sacrificing safety. This voltage level is, however, affected by temperature and the threshold is purposely set low enough to tolerate higher temperature charge. Tampering with a Li-ion charger is not recommended. |
||
|
|||||||||
|
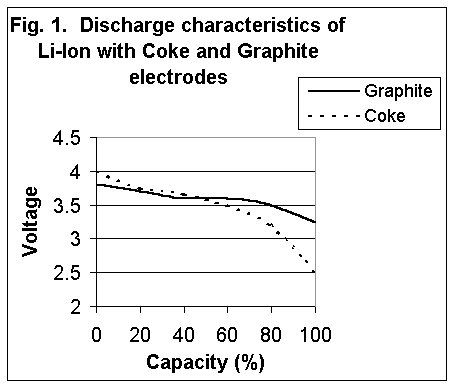
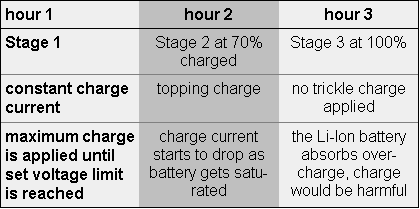
Chargers and battery analyzers that allow adjustments of the voltage thresholds should be set correctly when servicing coke- and graphite-based Li-ion batteries. Most battery manufacturers do not specify which version of Li-ion is used. If set incorrectly, a coke version will yield lower capacity readings, and a graphite will be slightly overcharged. At a moderate temperature, no damage occurs and the lower discharge voltage does not harm the graphite version battery. Figure 2 compares the Li-ion with the coke and graphite electrodes. The charge time of the Li-ion is about three hours, and the battery remains cool during charge. Full charge is attained after the voltage reaches the upper voltage threshold and the current drops and levels off to a low plateau. Increasing the charge current on a Li- ion charger does not significantly shorten the charge, especially on the coke type. Although the voltage peak is reached quicker with high current, the topping charge takes longer. Figure 3 shows the charge stages of a Li-ion charger. A more basic charge method terminates the charge as soon as the voltage level is reached. This charger is quicker and simpler than the two-stage charger, but only charges the battery to a 70 percent capacity level. Battery Analyzers for the Li-ion Battery analyzers are often associated with eliminating the memory phenomenon to restore lost capacity. What is the purpose of a battery analyzer when the Li-ion no longer exhibits memory? Conventional wisdom says that a new battery always performs flawlessly, yet many users have learned that a battery fresh from its shrinkwrap may not always meet the manufacturer's specifications. With a battery analyzer, non-performing batteries are identified, primed, and, if the capacity does not improve, returned to the vendor for warranty replacement. The typical life of a Li-ion is about 500 discharge/charge cycles. The loss of battery capacity occurs gradually and in many cases without the knowledge of the user. Although fully charged, the battery eventually regresses to a point where it may hold only half of its original capacity. The function of the battery analyzer is to identify these weak batteries in a fleet and "weed" them out. It only takes one bad battery to render a system unreliable. The problem is finding the faulty battery. Bad batteries tend to gravitate to the top and are picked more often than good ones. Here's why: The nonperformers are charged quicker and remain on "ready" longer than good batteries, making them more readily available to the unsuspecting user. Although the batteries are fully charged, they may hold only half the normal capacity. Similar to a fuel tank that is filled with crushed rock, the amount of fuel it can hold is less than a clean tank, even though the tank is full. In an emergency situation, the only batteries that are on "ready" may be those that are deadwood. With lives at stake, such a situation can have grave consequences. When choosing a battery analyzer, the user should make certain that all commonly used battery chemistries are supported, including the Li-ion chemistry. Units with a flexible platform that allow some adjustment of voltage settings are less prone to obsolescence when new battery types emerge that require different voltage thresholds. An example is the coke and graphite Li-ion chemistries, which require slightly different charge and discharge cutoff volt-.ges for effective testing. With new battery chemistries being introduced at a faster pace than ever, manufacturers of battery analyzers should offer firmware upgrades to service future batteries not yet commercially available. One such battery in development is the lithium polymer. Rather than using a nominal cell voltage of 3.6V common with the Li-ion, the lithium polymer will deliver 2.7 volts. Charge and discharge voltage thresholds and current ratings will also change. No known method is available to restore lost capacity of a Li-ion battery. In due time, procedures may become available that reactivate this battery type as well. This article originally appeared in "Radio Resource Magazine", and is re-printed with written permission from the editor |
||
|
|||||||||

|
|||||||||
|
|||||||||
|
|||||||||